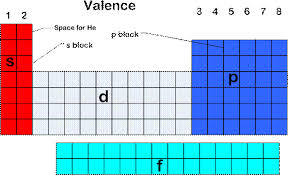
The Boron Group is the 13th group of the periodic table.
Boron is the 5th element of the periodic table. It is used to make green flares and has been used as an ignition source for rockets. It also combines to make insulation for homes. It can be made into flame retardant devices also.

Aluminum is the 13th element of the periodic table. It is the most abundant element on the earth crust but is never fount free in nature. Aluminum is used all over in many aluminum products from cans to foil. It also makes mirrors by placing it on glass. And it can be used to conduct electricity.

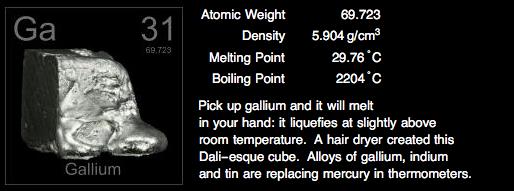
Gallium is the 31st element of the periodic table. It melts at room temperature making it useful in high temperature thermometers. It also has been used to help melt other alloys.
Indium is the 49th element of the periodic table. Indium is used as a lubricant in high speed motors. It can also be used to make mirrors.
Thallium is the 81st element of the periodic table. There is no use if it in its metallic state. But when combined with other substances it can create a low melting glass, and even rat poison, it was such a good poison it killed people in the houses, and was banned from houses. It was also used to murder people.